Home
We study early development using the zebrafish, as well as mouse, cell culture, and cavefish (Astyanax mexicanus) models.
A large component of our efforts are devoted to understanding mechanisms guiding the formation of blood and lymphatic vessels. The optical clarity and genetic and experimental accessibility of zebrafish make this a superb model organism for these studies. Our laboratory pioneered many of the key tools and resources used for vascular biology research in the zebrafish, including the widely used confocal microangiography method, a comprehensive atlas of the anatomy of the developing zebrafish vasculature, numerous vascular-specific transgenic fish lines, and methods for high resolution in vivo time-lapse imaging of zebrafish blood vessels. We have used these and other tools and resources to make a variety of seminal discoveries in the areas of vascular specification, differentiation, and patterning, including a novel pathway regulating arterial identity, a role for neuronal guidance factors in vascular patterning, a mechanism for vascular lumen formation in vivo, and identification and characterization of a lymphatic vascular system in the zebrafish. Cardiovascular-associated mortality is the leading cause of death in the western world, and many of the developmental processes we study are important in human congenital and acquired vascular diseases, as well as in cancer, heart disease, and ischemia.
We are also using cutting-edge molecular, cellular, genetic, transgenic, microscopic imaging, and next-gen sequencing approaches to study the meninges and meninges-associated cell types and the brain neurovascular interface. The meninges are complex, multilayered, highly vascularized tissues surrounding the brain that play a critical role in brain homeostasis and protection and are involved in a variety of brain pathologies. Our laboratory recently discovered that the zebrafish has a meningeal architecture very similar to that of mammals, and we are exploiting the advantages of the fish as a powerful new model for genetic and experimental dissection of this critical tissue. We are also interested in using the zebrafish to better understand the neurovascular interface throughout the brain, using the sophisticated genetic and imaging capabilities of the zebrafish.
Our laboratory has also recently developed an interest in studying the "genetics of epigenetics.” We have discovered novel epigenetic mechanisms regulating hematopoietic development and eye development, and we are currently carrying out the first large-scale forward-genetic screen in a vertebrate for tissue-specific epigenetic regulators using a novel transgenic reporter. This highly successful ongoing screen has resulted in the identification of a large number of new vertebrate epigenetic regulators, which we are now in the process of further characterizing and studying.
Learn more about our current research directions.
The Weinstein Laboratory is located in the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), at the National Institutes of Health (NIH) in Bethesda, Maryland. The NIH Bethesda campus is located just outside Washington, D. C., and is the largest biomedical research institution in the world, with approximately 1200 principal investigators. Learn more about the NIH.
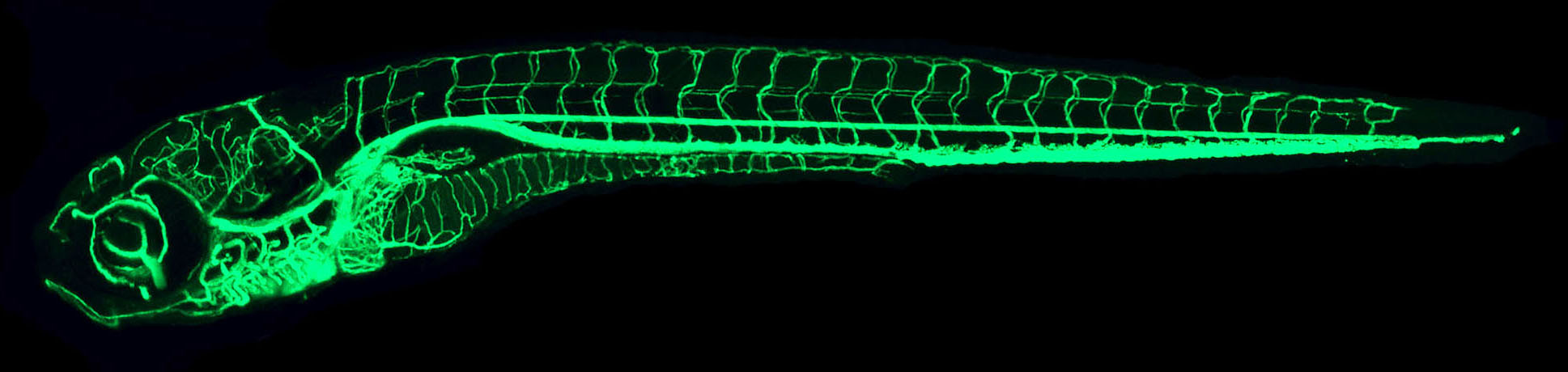
IMAGE: The confocal microangiography (intravascular injection of fluorescent dyes followed by confocal imaging) image above shows a lateral view of a 7 dpf whole zebrafish larva. The confocal microangiography technique was used by the Weinstein lab to compile a comprehensive atlas of vascular anatomy for the developing zebrafish.
Positions Available
Postdoctoral Position - Angiogenesis and Lymphangiogenesis
Postdoctoral Position - Studying the meninges and the neurovascular interface in the zebrafish
Postdoctoral Position - Epigenetics of Development
To apply to positions send a cover letter, full resume, and other materials as noted to weinsteb@mail.nih.gov and to amy.parkhurst@nih.gov.
 BACK TO TOP
BACK TO TOP