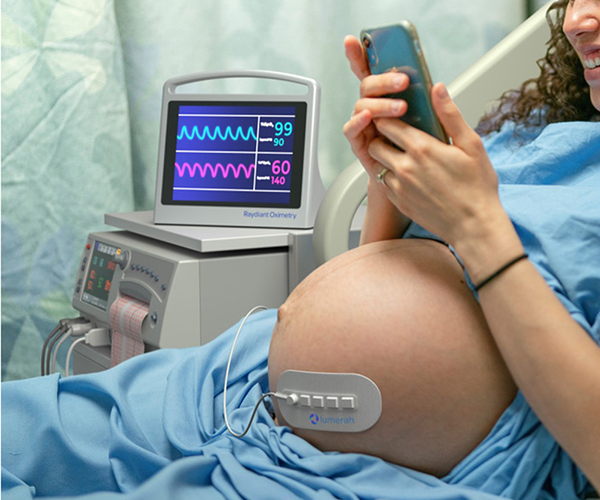
Lumerah concept illustration
Credit: Raydiant Oximetry, Inc.
For decades, healthcare providers have relied on cardiotocography to monitor fetal signs, like heart rate, during labor and delivery. However, this older technology has limitations that can lead to misinterpretations. For example, it can inaccurately suggest fetal hypoxemia (low blood oxygen levels), which causes unnecessary emergency Cesarean deliveries. It can also fail to detect hypoxic-ischemic encephalopathy, which occurs when the fetal brain does not receive enough oxygen, resulting in brain injury.
NICHD awarded small business funding to Raydiant Oximetry to support the development of LumerahTM, a non-invasive fetal pulse oximeter that detects fetal distress by directly measuring fetal arterial blood oxygen saturation. Lumerah has the potential to reduce rates of emergency Cesarean delivery and newborn neurological injury, as well as lower healthcare costs associated with childbirth. The technology can also serve as a research tool for better insights on pregnancy complications, including intrauterine growth restriction, preterm labor, and stillbirth.
In 2024, Raydiant Oximetry’s Lumerah device won first place in the RADx Tech Fetal Monitoring Challenge, which was co-sponsored by NICHD, the National Institute of Biomedical Imaging and Bioengineering, and the Bill & Melinda Gates Foundation. NICHD’s small business funds are currently supporting an early feasibility study in pregnant women at Eastern Virginia Medical School in Norfolk, Virginia.
 BACK TO TOP
BACK TO TOP