Home
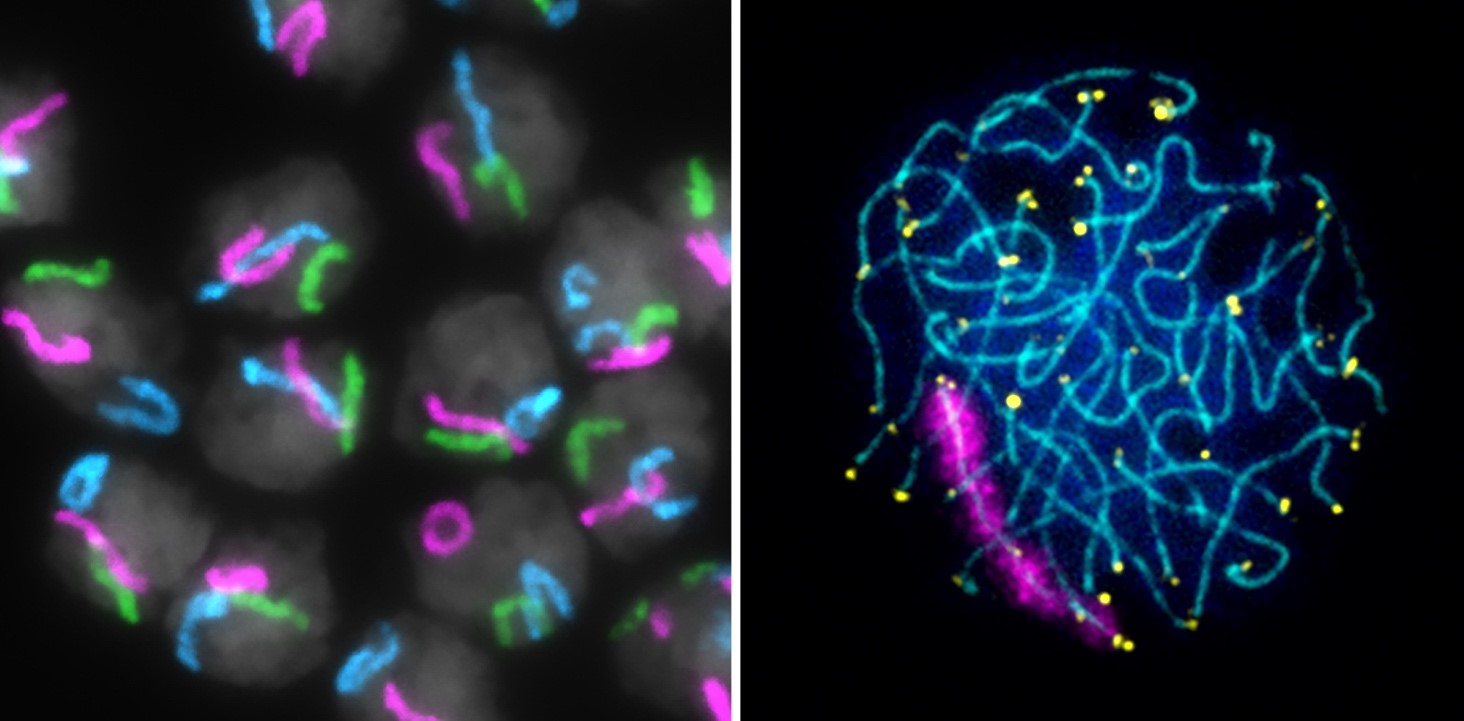
The goal of research in the Unit on Chromosome Dynamics is to understand the fundamental principles regulating inter-chromosomal communication in the germline and soma.
Precisely regulating inter-chromosomal interactions in all cell types is absolutely essential. In meiotic germline cells, homologs (maternal and paternal copies of the same chromosome) must come together and pair from end-to-end for accurate genetic recombination and chromosome segregation. Errors in chromosome segregation during meiosis can lead to reduced fertility, miscarriages, or chromosomal disorders in progeny, such as Down Syndrome or Turner Syndrome. While pairing must be robust in meiotic cells, interactions between homologs in somatic cells are tightly regulated and occur only under specialized circumstances. For example, somatic pairing in mammals facilitates the inter-chromosomal communication required for X-chromosome inactivation and DNA repair. Yet, how homologs find each other in 3D nuclear space and distinguish “self” from “non-self” remains one of the biggest mysteries in chromosome biology.
Understanding this central aspect of genome organization will not only provide insights into how disruption of these processes can result in genome instability, infertility, and cancer, but will provide many potential new avenues for therapeutic targets for genome instability-related human diseases. In a world where we know how homologs find each other for meiotic recombination, we could harness this information to generate strategies to inhibit pairing in tumors to reduce DNA repair efficiency for more robust DNA damage-based cancer treatments. Similarly, methods to promote pairing could have profound impacts on treatments for infertility, as premature loss of pairing is one of the leading causes of human aneuploidies.
To dissect the mechanisms regulating homolog pairing and inter-chromosomal communications, our lab employs the Oligopaint fluorescence in-situ hybridization (FISH) technology, live imaging, and high-throughput genomics-based assays in insect and mammalian model systems.





 BACK TO TOP
BACK TO TOP