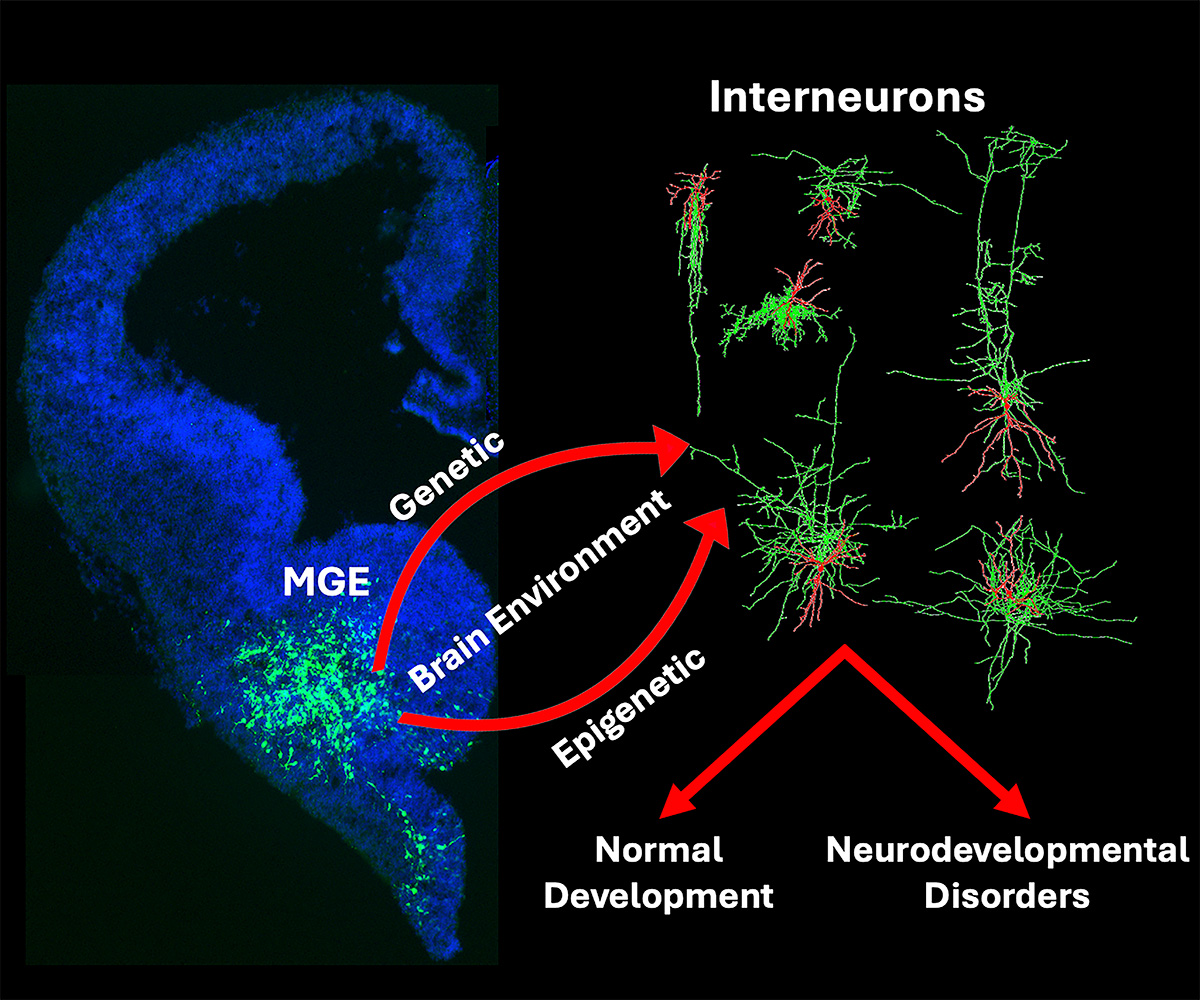
Diagram modeling how genetic and epigenetic mechanisms influence the development of interneurons.
Credit: Petros Lab
Inhibitory GABAergic interneurons are a heterogenous cell population that are critical for establishing the proper excitatory-inhibitory balance in the brain. Interneuron dysfunction is associated with a variety of brain disorders, such as epilepsy, schizophrenia and autism. While mutations in specific genes can lead to disease, there is growing evidence that disruption of normal gene regulatory mechanisms (i.e., epigenetics) is associated with many neurodevelopmental disorders. The methyltransferase Ezh2 is critical for repressing gene expression during development, and humans with EZH2 variants are often diagnosed with Weaver syndrome, a complex disease with intellectual disability. To explore the role of Ezh2 in interneuron development, the Petros Lab removed Ezh2 specifically in the medial ganglionic eminence (MGE), the source of many cortical interneurons. Loss of Ezh2 in MGE-derived interneurons led to a variety of deficits highlighted below:
- Ezh2 mutant mice have fewer interneurons in the cortex and hippocampus compared to WT mice. Additionally, there was shift in interneuron subtype fates, with a reduction of parvalbumin-expressing and an increase in somatostatin-expressing interneurons in the mutant mice.
- While the electrophysiological properties of these interneurons were normal, parvalbumin-expressing interneurons lacking Ezh2 had significantly longer and more complex axonal arbors; possibly a compensation mechanisms for the overall reduction of this interneuron subtype.
- Single cell sequencing experiment in the MGE of WT and Ezh2 mutant mice revealed changes in both gene expression profiles and chromatin accessibility that were predictive of the observed changes in cell fate.
- Analysis of histone methylation patterns demonstrated that some genomic loci were more susceptible to loss of Ezh2 while other loci were more resistant. This finding of differential susceptibility at specific genomic loci could have important implications for how epigenetic perturbations preferentially target certain genes or genomic loci.
Reference
Rhodes CT, Asokumar D, Sohn M, Naskar S, Elisha L, Stevenson P, Lee DR, Zhang Y, Rocha PP, Dale RK, Lee S, and Petros TJ. Loss of Ezh2 in the medial ganglionic eminence alters interneuron fate, cell morphology and gene expression profiles Frontiers in Cellular Neuroscience DOI: 10.3389/fncel.2024.1334244 (2024)
Learn more about the Neurosciences Affinity Group: https://www.nichd.nih.gov/about/org/dir/affinity-groups/neurosciences
 BACK TO TOP
BACK TO TOP