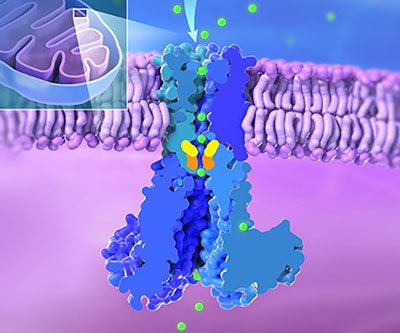
Cross-section of MRS2 channel illustrating translocation of magnesium ions (green) across the inner mitochondrial membrane. The R332 and M336 gates regulating magnesium ion transport are colored in orange and yellow respectively. Inset shows one mitochondrion.
Credit: Ethan Tyler, NIH Medical Arts; Louis T.F. Lai, Ph.D., Matthies Lab, NICHD
Magnesium, an essential nutrient obtained through the diet, is required for many biological processes and serves as a co-factor for hundreds of enzymes. Dysregulation of magnesium can result in various diseases, from cardiovascular conditions to cancer. Mitochondria—the energy producers in cells—obtain magnesium through the Mitochondrial RNA Splicing 2 (MRS2) channel. However, the details of how magnesium transport is regulated through this channel have remained unclear.
- Researchers from the Matthies Lab used cryo-electron microscopy to unveil the structure of human MRS2 in the presence and absence of magnesium. They found that MRS2 is a five-subunit structure, or pentameric channel, with one central pore that magnesium passes through. Two key areas, called R332 and M336, serve as gates to regulate entry of magnesium.
- In addition to the known “ion selectivity filter” at the entrance of the pore, which pulls in positively charged magnesium ions, the researchers found a variety of negatively charged residues, which can direct the magnesium ions.
- Structural analysis revealed several features involved in regulating the channel’s function. They identified interactions between R116 and E291 across the channel’s soluble domains that play a role in channel activity.
- Their findings provide foundational knowledge on a process needed for healthy muscles and bones, nerve function, and immunity. Such knowledge can benefit researchers who study conditions or diseases in which magnesium is dysregulated in the body.
NICHD authors of the paper include Louis Tung Faat Lai, Jayashree Balaraman, Fei Zhou, and Doreen Matthies.
Learn more about the Physical Biology and Medicine group: https://www.nichd.nih.gov/about/org/dir/affinity-groups/PBM
 BACK TO TOP
BACK TO TOP