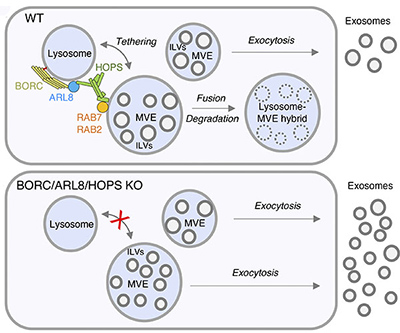
In typical cells (top panel), BORC, ARL8, and HOPS help tether lysosomes to multivesicular endosomes (MVEs). The contents of the resulting lysosome-MVE hybrids are degraded. In cells lacking BORC, ARL8, or HOPS (bottom panel), tethering and fusion of lysosomes and MVEs does not occur. The contents of the MVEs are then released as exosomes.
Credit: Bonifacino Lab
Exosomes are very small sacs that are secreted from cells to dispose of undegraded materials and assist with cell-cell communications. They also are being explored as disease biomarkers and potential treatments. One limitation to their therapeutic use is the low yields of exosomes that can be obtained from cultured cells under standard conditions. Recent research from the Bonifacino Lab seeking to understand the factors that influence exosome secretion suggests a strategy to boost exosome yields.
One major source of exosomes is multivesicular endosomes (MVEs), which help transport proteins marked for degradation to lysosomes, the cell’s waste-disposal system. MVEs carry small, protein-containing sacs known as intraluminal vesicles (ILVs). Often, MVEs fuse with lysosomes, delivering the ILVs for degradation. But sometimes, the contents of MVEs are released out of the cell as exosomes. The factors that determine which fate MVEs undergo remain unknown.
The Bonifacino Lab investigated the roles of the proteins BORC and ARL8, which had been implicated in determining MVE fate, and some of their effectors in human cell culture experiments. They found that cells engineered to lack ARL8 or components of the BORC or HOPS protein complexes secreted more exosomes. This higher exosome secretion is due to the diminished ability of MVEs to tether to and fuse with lysosomes. Thus, using cell lines engineered to lack fully functional BORC, ARL8, or HOPS may offer a convenient strategy to boost exosome production for biotechnology applications. The results also suggest that exosome secretion may be enhanced in diseases in which lysosome functions are not properly regulated, highlighting the potential of exosomes as disease biomarkers.
NICHD co-authors of the paper include Ganesh Vilas Shelke, Chad D. Williamson, and Michal Jarnik.
Learn more about the Cell and Structural Biology group: https://www.nichd.nih.gov/about/org/dir/affinity-groups/CSB
 BACK TO TOP
BACK TO TOP