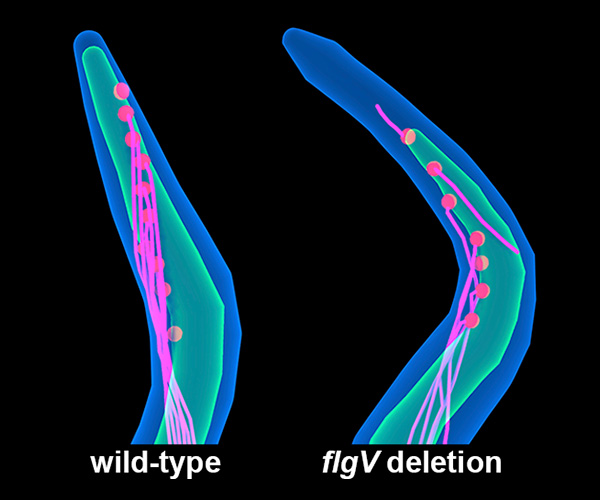
“Whiplike” flagella are shorter and impaired in a strain of Lyme-disease-causing Borrelia burgdorferi bacteria lacking the flgV gene (flgV deletion), compared to typical B. burgdorferi (wild-type). Depicted are the outer membrane (blue), inner membrane (green), flagellar basal bodies (red), and flagellar filaments (pink).
Credit: Liu Lab (Yale University) and Adams Lab (NICHD)
Lyme disease results from a bacterial infection that occurs when a human is bitten by a tick infected with Borrelia burgdorferi. Movement of B. burgdorferi bacteria from ticks to mammalian hosts is essential for infection. Bacteria generally require complex whiplike structures called flagella to move through environments. Butthe steps to build and control B. burgdorferi flagella function are poorly understood. Work led by Philip Adams, Ph.D., and other NICHD researchers provides insight into the synthesis and function of B. burgdorferi flagella.
- Researchers established that the B. burgdorferi gene flgV is an important flagellar component that helps control assembly of flagella.
- Strains of B. burgdorferi engineered to lack flgV produced fewer and shorter flagella and were defective in movement and cell division, compared to strains producing the FlgV protein.
- B. burgdorferi lacking flgV were able to survive and replicate in ticks. However, other experiments showed that flgV is important for infection and dissemination of the bacteria in mice.
- Overall, the work establishes FlgV as an important modulator of flagellar assembly and finds that even slight changes to the number or length of flagella can have noticeable consequences for the infectivity of B. burgdorferi.
- Understanding how B. burgdorferi traverse, replicate, and divide inside their hosts will provide valuable insights into the biology of Lyme disease and open opportunities to develop treatments.
Reference
Zamba-Campero M, Soliman D, Yu H, Lasseter AG, Chang YY, Silberman JL, Liu J, Aravind L, Jewett MW, Storz G, Adams PP. Broadly conserved FlgV controls flagellar assembly and Borrelia burgdorferi dissemination in mice. Nature Communications DOI: 10.1038/s41467-024-54806-w (2024)
Learn more about the Cell and Structural Biology group: https://www.nichd.nih.gov/about/org/dir/affinity-groups/CSB.
 BACK TO TOP
BACK TO TOP