Finding promises to improve drug design for common forms of cancer
Credit: Jeremy Swan, NICHD/NIH
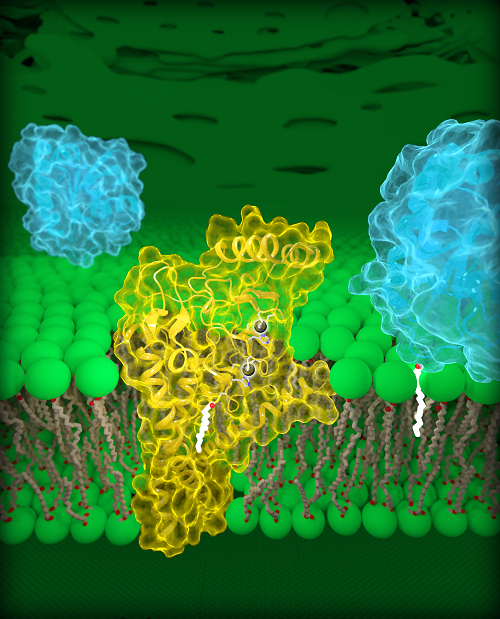
The first three-dimensional structure of DHHC proteins—enzymes involved in many cellular processes, including cancer—explains how they function and may offer a blueprint for designing therapeutic drugs. Researchers have proposed blocking DHHC activity to boost the effectiveness of first-line treatments against common forms of lung and breast cancer. However, there are currently no licensed drugs that target specific DHHC enzymes. The study, led by researchers at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), appears in the latest issue of Science. NICHD is part of the National Institutes of Health.
DHHC enzymes, also called palmitoyltransferases, modify other proteins by attaching to them a chain of lipids, or fatty acids, of varying lengths. This modification, called palmitoylation, can change many properties of a target protein, such as its structure, function and location within a cell. Researchers estimate that nearly 1,000 human proteins undergo palmitoylation, including epidermal growth factor receptors (EGFRs). A well-known EGFR is HER2, which is overactivated in aggressive forms of breast cancer. EGFRs can also be overactivated in colon cancer, and non-small cell lung cancer, the most common type of lung cancer.
The current study details the structures of a human DHHC enzyme, DHHC20, and the zebrafish version of another DHHC enzyme, DHHC15. Importantly, DHHC20 is the enzyme that palmitoylates EGFR. Previous studies have shown that blocking DHHC20 makes cancer cells more vulnerable to existing FDA-approved treatments that target EGFR. Therefore, understanding the structure of DHHC20 may be important for treating EGFR-driven cancers.
“Mutations in DHHC enzymes are associated with various cancers and neurological disorders,” according to Anirban Banerjee, Ph.D., the study’s lead author and head of NICHD’s Unit on Structural and Chemical Biology of Membrane Proteins. “Our study offers a starting point for developing DHHC20 inhibitors that may aid in treatment of common cancers and advance the field of protein palmitoylation.”
Dr. Banerjee and colleagues identified a structural component, a cavity, of DHHC20 that influences the length of its lipid chain. Mutations that altered the relative size of this cavity caused DHHC20 to use shorter or longer lipid chains, which presumably changes the effects of palmitoylation on a target protein. The researchers propose that the structure of this site explains why different DHHC enzymes use certain lipid chains to modify the functions of other proteins. It also offers insight on how multiple enzymes work together in states of health and disease.
Funding was provided by NICHD and the National Institute of Neurological Disorders and Stroke. Additional support was provided by the National Institute of General Medical Sciences, the National Cancer Institute, the NIH Office of Research Infrastructure Programs, and the U.S. Department of Energy.
ARTICLE:
Rana MS, Kumar P, Lee CJ, Verardi R, Rajashankar KR, and Banerjee A. Fatty acyl recognition and transfer by an integral membrane S-acyltransferase. Science DOI: 10.1126/science.aao6326 (2018)
###
About the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): NICHD conducts and supports research in the United States and throughout the world on fetal, infant and child development; maternal, child and family health; reproductive biology and population issues; and medical rehabilitation. For more information, visit NICHD’s website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.nih.gov.

 BACK TO TOP
BACK TO TOP