Newborn screening programs across the United States currently screen 4 million infants each year. This public health program detects treatable disorders in newborns, allowing treatment to begin often before symptoms or permanent problems occur. Newborn screening not only saves lives, but can also improve the health and quality of life for children and their families.
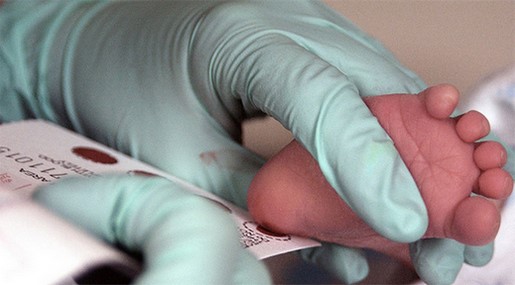
Within the first 24 to 48 hours after birth, babies undergo a simple heel stick and a few drops of blood are collected on a special paper card. Health care providers then test those blood spots for a number of congenital disorders (conditions that are present at birth). Infants are also screened for hearing disorders and for other serious heart problems using methods other than dried blood spots.
When a screen is positive for any condition, the newborn's parents are informed that their infant has an "out-of-range" test result for one of the conditions. But a positive screening test does not mean the infant has a disorder. Additional testing is needed to confirm and diagnose the condition.
Only about one out of 300 newborns is later diagnosed with a treatable condition. Once the condition is confirmed, the infant is referred to an appropriate specialist so that treatment can begin as early as possible.


 BACK TO TOP
BACK TO TOP